Continuous External Fetal Monitoring Involves the Use of a Spiral Electrode
The initial description of the fetal heart rate (FHR) apparently dates to Marsac's report in 16501. As early as 1893, Von Winckel2 suggested that a FHR greater than 160 beats per minute (bpm) or less than 100 bpm is indicative of fetal distress. Intrapartum fetal surveillance by intermittent auscultation of the FHR continued to evolve early in the 20th century and, in fact, had become standard practice. During the late 1940s and 1950s, however, it became obvious that counting the FHR over time and calculating the mean value was an unsatisfactory method for detecting potential fetal compromise.3
In an attempt to improve on intermittent auscultation as a means of fetal surveillance, continuous intrapartum EFM was introduced into clinical practice in the early 1970s in the United States. Several retrospective studies suggested that fetuses monitored with continuous EFM experienced significantly improved outcomes compared with fetuses monitored with intermittent auscultation.4 The practice of continuous EFM was embraced enthusiastically as an accurate, objective, and reproducible technique that provided evidence of the early fetal hypoxemia or acidemia that typically precedes permanent fetal neurologic damage.5 , 6 Unfortunately, this technology was introduced and assimilated into wide clinical practice before a prospective, randomized analysis of this hypothesis.
To date, 13 published randomized controlled trials have compared the efficacy and safety of routine continuous EFM versus intermittent auscultation. An updated meta-analysis of these studies appeared in January 2001, and the reviewers concluded that the only clinically significant benefit of routine continuous EFM was a reduction in neonatal seizures (relative risk [RR] 0.51, 95% confidence interval [CI] 0.32–0.82).7 Continuous EFM was associated, however, with an increased rate of cesarean delivery (RR 1.41, 95% CI 1.23–1.61) and operative vaginal delivery (RR 1.20, 95% CI 1.11–1.30). There seems little question that randomized prospective studies of continuous EFM with low- and high-risk pregnancies have not confirmed the benefit that was suggested by earlier retrospective reports.8 These findings have been confirmed by a recent meta-analysis, which showed that for every 628 women monitored, 1 neonatal seizure would be avoided and 11 cesarean sections would be done unnecessarily.9 Obviously, with respect to the high stakes for patients and obstetricians, a screening test with a high sensitivity (even at the cost of a low specificity) is desireable. Although meta-analyses have failed to confirm improvements in outcome with external fetal monitoring, this is likely because even mildly abnormal fetal tracings are quickly acted upon and result either in delivery or intrauterine resuscitation.
Freeman, one of the pioneers of fetal assessment with heart rate monitoring (both antepartum and intrapartum), lamented, "Clearly, the hoped-for benefit from intrapartum (continuous) electronic fetal monitoring has not been realized."6 In a more recent discussion of the limited efficacy of intrapartum EFM,10 Parer and King offered a number of factors potentially contributing to these disappointing results, including unrealistically high expectations, the lack of standardization of definitions of FHR patterns, the poor reliability of FHR interpretation, and the failure to show the validity of FHR monitoring in detecting fetal asphyxia. Under most clinical circumstances, however, the only alternative to continuous intrapartum EFM is intermittent auscultation by a nurse providing one-on-one patient care. For most labor and delivery units, this level of commitment of nursing resources simply is not possible given current (and likely future) financial constraints as well as nursing personnel limitations. These practical considerations, as well as the current medicolegal climate in the United States, which demands virtually continuous documentation of fetal well-being, dictate that the primary modality for intrapartum fetal surveillance in most labor and delivery units is continuous EFM, with a much smaller number of patients being monitored with intermittent auscultation.
A recent study by Larma et al.11 set out to determine the sensitivity, specificity, positive and negative predictive values of fetal heart rate monitoring for neonatal hypoxic-ischemic encephalopathy. They looked at parameters of the fetal heart tracing such as bradycardia, decreased variability, nonreactivity, and looked at all three variables combined. Interestingly, they found that decreased variability was the most predictive aspect of the FHT with a sensitivity of 92.3%, a specificity of 61.7%, positive predictive value of 2.7%, and a negative predictive value of 82.9%. When looking at all three parameters combined, they found that fetal heart rate monitoring had a sensitivity of 7.7%, specificity of 98.9%, positive predictive value of 50%, and a negative predictive value of 88.9%. Therefore, as a screening test, decreased variability, with it's reasonably high sensitivity at 92.3% has the best performance.
The above studies should not be interpreted as proof that intrapartum fetal monitoring (either intermittent auscultation or continuous EFM) is without benefit. These trials examined the relative benefits of alternative forms of intrapartum monitoring: they did not compare the perinatal outcome of monitored versus unmonitored fetuses. Thus, although no method of intrapartum fetal surveillance clearly is superior to all others, a substantial body of literature confirms that intrapartum fetal monitoring (intermittent or continuous), although a relatively poor diagnostic test, is a reliable screening test for the confirmation of fetal well-being. The American College of Obstetricians and Gynecologists (ACOG) offered the following guidelines in 2005.8 Level A Recommendations: The false positive rate of EFM for predicting adverse outcomes is high. The use of EFM is associated with an increased rate of operative intervention. The use of EFM does not reduce cerebral palsy rates. Amnioinfusion reduces the need to proceed with an emergent cesarean section and should be considered in the context of severe variable decelerations. Level B recommendations: The labor of patients with at high risk for maternal or fetal complications should be monitored continuously. Reinterpretation of the FHR tracing, particularly when the outcome is known, is not reliable. The use of fetal pulse oximetry in clinical practice can not be supported at this time.8
Uterine Activity
Various intrapartum fetal monitoring systems are available from many manufacturers. Because new models and modifications are introduced into clinical practice on a continual basis, this discussion focuses on the general characteristics common to all intrapartum EFM programs rather than the specific systems that are available.
External monitoring of uterine contractile activity involves the use of a pressure-sensitive plate called a tocodynamometer. The only information provided by the external tocodynamometer is the frequency and duration of uterine contractions. A tocodynamometer provides only very limited information about the relative strength and intensity of uterine contractions. The device is placed over the uterine fundus and stabilized with an adjustable elastic belt. Coupling jelly is not required; in fact, its use may damage the transducer. Uterine activity is recorded by interpreting the movement of the anterior abdominal wall; this movement occurs at the time of uterine contractions. The device functions essentially as an electromechanical hand and should be placed at the point of maximal abdominal wall displacement, usually in the midline over the fundus.
Information about the intensity and strength of uterine activity is most accurately provided by direct monitoring obtained with an intrauterine pressure catheter. Most commercially available systems include a stiff catheter guide that is inserted just beyond the fetal presenting part and no further. The pressure catheter is advanced into the uterine cavity, and the catheter guide is removed. The appropriate depth of insertion can be determined by a mark on the catheter that should be at the level of the introitus after insertion. The catheter should be inserted posterior to the fetal presenting part. If the presenting part is well engaged in the pelvis, posterior insertion may be impossible, and anterior insertion may be attempted. Lateral insertion is discouraged because lateral perforation may precipitate uterine artery compromise with its attendant complications, including broad-ligament hematoma. Currently available catheters interface with the fetal monitor via a manufacturer-specific reusable cable. The set-up and calibration process are straightforward, and most systems incorporate a lumen into the catheter to permit simultaneous monitoring of intrauterine pressure as well as amnioinfusion. Various commercial models of intrauterine pressure catheter are available, including the Intran™ from Utah Medical Products12 and the Koala™ from Clinical Innovations™.13
Fetal Heart Rate
In contemporary practice, continuous intrapartum assessment of FHR by external monitoring involves the use of a continuous-wave Doppler ultrasound device. The Doppler ultrasound transducer is empirically located on the maternal abdomen at the site where the fetal cardiac motion is most easily heard. A water-soluble jelly is used, and the transducer is held in place with an adjustable elastic belt. Fixation of the transducer with elastic tape may be preferable because it allows signal detection and recording despite maternal movement. Current external FHR monitors provide an approximation of the FHR that represents a highly processed signal generated by electronic autocorrelation software. Because the FHR is not being recorded instantaneously, external monitoring does not necessarily provide an opportunity to accurately assess variability. The heart rate variability that is apparent on external FHR monitor strips is modestly exaggerated (due to the artifact inherent in the process of signal detection) compared with FHR recordings obtained by internal or direct methods. In addition, external FHR monitoring is much more subject to artifact resulting from changes in maternal position, maternal obesity, and fetal movement.
These limitations of external FHR monitoring must be balanced against several other considerations. First, valuable information regarding fetal status may be obtained in the absence of ruptured membranes or any significant cervical dilatation. Second, fetal monitoring using this technique can routinely be initiated with monitor placement by the nursing staff. Finally, assuming that artifact is minimized, external FHR tracings usually are representative of what would be obtained with internal monitoring.
Internal or direct FHR monitoring is performed with the attachment of a spiral fetal electrode to the presenting part to obtain electrocardiographic data directly. In common usage, the spiral electrode is referred to as a scalp electrode because cephalic presentations greatly outnumber breech presentations. However, the spiral electrode works equally well regardless of the presenting part to which it is attached. The electrode is enclosed within a stiff plastic insertion sheath for application. Before application of the electrode, membranes must be ruptured, either spontaneously or surgically, and the cervix must be dilated at least 1 to 2 cm. Some knowledge of the fetal presentation is required before placement of the spiral electrode to obviate the possibility of application to the fetal face, eyelid, or other vulnerable facial structure. After application, the insertion sheath is removed, the wire is attached to the distal portion of a cable attached to the patient's leg, and the proximal portion of the cable is connected to the monitoring system.
The fetal electrode captures fetal electrocardiac activity, and the software extracts the R wave. The interval between R waves is measured, and the R–R interval is transformed into the instantaneous FHR, which is printed on the FHR monitor strip. This heart rate determination is not the result of a highly processed signal or averaging technique, but rather a direct measurement that allows accurate assessment of variability.
The frequency of use of internal monitors (both the fetal scalp electrode and IUPC) differs by centers, with some centers preferring to use external monitors when they are able to get reasonably good data and other centers preferring to use internal monitors more commonly. There is no data to suggest that mode of monitoring (whether internal or external) has a significant effect on outcomes, therefore, clinicians should use monitoring in the manner that they are the most comfortable with.
In the United States, both external and internal data (uterine contraction and FHR) are recorded on heat-sensitive graph paper at a rate of 3 cm/minute. Vertical lines appear every 10 seconds, with heavier demarcations obvious every minute. The bottom third of the graph paper shows uterine activity data. A horizontal scale of 0–100 mmHg covers 4 cm of display. The upper two-thirds of the record displays FHR data, with a scale of 30–240 bpm covering 7 cm of the monitor strip. If the fetal monitor strip is exposed to heat (e.g., during overhead projection for teaching conference purposes), the paper may become blackened.
To some extent, sophisticated interpretation of FHR monitor strips is an art rather than an exact science. Because of this subjective and sometimes controversial nature of FHR interpretation, the National Institute of Child Health and Human Development convened a workshop to develop standardized, unambiguous, quantitated definitions for interpretation of intrapartum FHR tracings. By so doing, the predictive value of intrapartum EFM can be assessed in prospective trials in a more reproducible manner. It is hoped that this initiative will ultimately yield data that facilitate FHR monitor strip interpretation in an evidence-based manner. Interested readers should consult the workshop's report to review the detailed, standardized definitions published in 1997.14 Since that time, the Joint Commission on Accreditation of Healthcare Organizations (JACHO) endorsed the NICHD guidelines in 2004 and ACOG endorsed the NICHD guidelines in 2005.
Currently, intrapartum EFM is best viewed as a screening test to confirm fetal well-being rather than a diagnostic test confirming fetal compromise. Clinical factors, stage of labor, evolution of a particular pattern, and the characteristics of the entire FHR monitor strip must influence any given interpretation. No single method of classification or interpretation, regardless of its complexity, can be expected to provide clear guidelines for the management of borderline or difficult-to-classify patterns. Finally, because the status of the fetus during the labor process is dynamic, the data produced by intrapartum EFM are continuous, and the FHR strips must be examined on an ongoing basis.
Analysis of intrapartum EFM strips must be approached in an organized, systematic manner to optimize the value of the information obtained, to avoid overwhelming the practitioner with the volume of data obtained, and to prevent the practitioner from overlooking important clinical events. In general, the following six characteristics should be included in any thorough analysis:
- The technical quality of the monitor strip, to ensure that artifact does not make interpretation impossible (inadequate data must not be interpreted; instead, the quality of the data obtained must be improved)
- Determination of whether external or internal monitoring methods are being employed
- Evaluation of the FHR baseline rate
- Analysis of the FHR reactivity (external monitor) or beat-to-beat variability (internal monitor)
- Inspection for the presence of FHR accelerations
- Consideration of periodic FHR decelerations associated with uterine contractions
During the analytic process, multiple abnormalities in a particular FHR tracing are more significant than isolated findings. Fetal scalp pH determination or evoked fetal response to scalp stimulation or vibroacoustic stimulation may be helpful in the accurate assessment of fetal status in the face of nonreassuring FHR data.
Uterine Contractions
The intrauterine baseline pressure is defined simply as the pressure extant between contractions. An approximation of this variable can be obtained only by means of an intrauterine pressure catheter monitoring system. The upper limits of normal baseline intrauterine pressure may be defined according to the stage of labor: for latent labor, less than 5 mmHg; for the active phase, less than 12 mmHg; and for the second stage of labor, less than 20 mmHg. Baseline pressures greater than these levels may represent uterine hypertonicity and sometimes are associated with abruptio placentae, uterine hyperstimulation with oxytocin, fetal malposition (e.g., occiput posterior and occiput transverse), or cephalopelvic disproportion.15
Although no uniform definition exists, uterine tachysystole has been defined as six contractions in 10 minutes in consecutive 10-minute intervals. Uterine hyperstimulation has been defined as a similar contraction frequency or a series of contractions lasting more than 2 minutes in combination with potentially worrisome FHR changes (e.g., late decelerations or fetal bradycardia).16 , 17 If tachysystole occurs in association with an elevation of baseline intrauterine pressure, the risk of uterine hyperstimulation is increased significantly. Similarly, if oxytocin induction or augmentation becomes complicated by tachysystole, the oxytocin should be discontinued or the rate of infusion decreased, at least temporarily, to avoid uterine hyperstimulation.
The graphic representations of normal uterine contractions on intrapartum EFM strips tend to be bell-shaped (i.e., symmetric), with a frequency in active labor of approximately one contraction every 2–3 minutes, as measured from the beginning of one contraction to the beginning of the next. Experienced clinicians recognize, however, that some patients may have an irregular contraction pattern throughout labor with an entirely normal progress of labor. The potential issue of apparently inadequate uterine contraction frequency is of no concern as long as satisfactory labor progress is occurring.
Similarly, there is no simple relationship between the intensity of uterine contractions and the rate of cervical dilatation. External tocodynamometry provides no information regarding the strength or intensity of uterine contractions. Even with an intrauterine pressure catheter system, the deflection on the intrapartum EFM strips is not necessarily an accurate indicator of the quality of labor. Nonetheless, contractions that occur less frequently than every 5 minutes and have a peak amplitude of less than 25 mmHg seldom are associated with a normal labor curve, particularly in the active phase of labor. Once again, however, a detailed analysis of the frequency and intensity of uterine contractions is necessary only when the rate of cervical change is abnormal.
Fetal Heart Rate Baseline Value
The FHR baseline value is defined as the approximate heart rate between uterine contractions. The normal range is 110–160 bpm. Baseline rates below this range (less than 110 bpm) are considered bradycardia, whereas fetal baseline tachycardia is defined by a FHR greater than 160 bpm for two contraction cycles or longer than 5 minutes. Deviations from this normal baseline range are not necessarily indicative of fetal compromise. For example, a baseline rate of 100–110 bpm, if not associated with other FHR abnormalities or a significant decrease from a previously higher baseline rate, may be associated with an entirely reassuring intrapartum EFM strip.
Severe FHR baseline bradycardia (less than 100 bpm) is more likely to be associated with fetal jeopardy, although fetal congenital heart block can produce an identical FHR tracing that is not associated with acute compromise, and should be excluded when patients present with a fetal bradycardia in the mid- or early third trimester prior to proceeding with an emergency delivery, when practical. Clearly, there are instances where there is evidence for another cause of fetal bradycardia (such as vaginal bleeding, cord prolapse, etc.), where delay may compromise the fetal status. Delivery should not be delayed in these cases. Also, there are conditions where the technology (such as M-mode or Doppler ultrasound) or the expertise to exclude congenital heart block as a cause of fetal bradycardia are not available. It may be similarly unwise to delay delivery in these cases. Finally, near term, the additional risk of delivery is low enough that the risk-benefit ratio may favor delivery over delay.
Fetal tachycardia may not be as clear an indicator of potential fetal compromise as severe baseline bradycardia. Fetal baseline tachycardia is classified as mild from 160 to 180 bpm and as severe when greater than 180 bpm. The differential diagnosis for the etiology of fetal tachycardia includes fetal compromise, maternal fever, prematurity, fetal infection, fetal arrhythmia (more common with fetal heart rates greater than 200 bpm), maternal thyrotoxicosis, fetal anemia, and maternal drug ingestion (prescribed or illicit). After excluding these potential confounding etiologies, and if combined with other FHR abnormalities, the index of suspicion for fetal compromise should be increased. For example, the combination of fetal tachycardia with diminished variability and late decelerations would be nonreassuring, as would the combination of fetal tachycardia with severe variable decelerations. The measures for intrauterine resuscitation described above for fetal bradycardia can also be effective interventions for fetal tachycardia. In general, regardless of the type of nonreassuring FHR pattern, steps should be taken (and documented) to optimize fetal status.
Fetal Heart Rate Variability
The presence of normal FHR variability is a reassuring sign of fetal well-being and is attributed to the interaction of an intact fetal sympathetic and parasympathetic nervous system. Fetal heart rate variability is defined as fluctuations in the baseline FHR of two cycles per minute or greater.14 These fluctuations are visually quantitated as the amplitude of the peak to trough (in bpm) as below:
Absent: amplitude range undetectable
Minimal: amplitude range more than undetectable but less than 6 bpm
Moderate: amplitude range 6–25 bpm
Marked: amplitude range more than 25 bpm
Accurate assessment of variability requires direct EFM (i.e., analysis of instantaneous FHR data via scalp electrode) because external Doppler techniques yield a "processed" signal (via autocorrelation) that may exaggerate the magnitude of variability present. Although satisfactory variability is very reassuring, its absence is not necessarily associated with fetal compromise. Diminished variability (Fig. 1) may be associated with maternal narcotic use, administration of other drugs (e.g., diazepam, sodium thiopental, possibly magnesium sulfate), fetal neurologic dysfunction that predates labor, fetal baseline tachycardia (especially when associated with B-mimetic tocolytic therapy), or, most commonly, fetal sleep state.
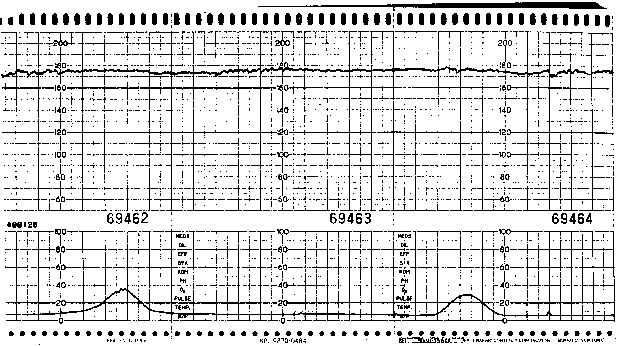 Fig.1. Fetal heart rate pattern with baseline tachycardia and minimal variability.
Fig.1. Fetal heart rate pattern with baseline tachycardia and minimal variability.
Accordingly, the presence of diminished variability in the absence of other FHR abnormalities, or decreased variability that is readily attributable to a known cause rarely indicates fetal compromise. In contrast, absent variability coupled with late decelerations is nonreassuring and may be an indication for digital scalp stimulation (or vibroacoustic stimulation) to evoke FHR accelerations or fetal scalp sampling for pH determination to provide evidence of fetal well-being. Clearly, accurate assessment of the intrapartum fetal status requires an overview of the entire clinical scenario, not the simple categorization of isolated, perhaps even transient, FHR tracing characteristics.
Infrequently, the baseline variability may be increased (greater than 25 bpm). This so-called saltatory pattern may be observed early in labors complicated by fetal malposition or disproportion and on occasion deteriorates into a pattern characterized by significant variable decelerations.18 The ultimate clinical significance of the saltatory pattern remains unclear, and operative intervention for this finding in isolation seems unwarranted.
Also infrequent is the sinusoidal FHR pattern. Characterized by the smooth, undulating oscillation of the baseline (mimicking a sine wave), short-term variability is absent in this pattern. The amplitude of the long-term baseline variability ranges from 5 to 15 bpm, and the frequency of the cycles generally is three to five cycles per minute. This FHR pattern must be present for at least 10 minutes to avoid overdiagnosing this rare pattern, which classically is associated with severe fetal anemia (e.g., secondary to isoimmunization).19 The sinusoidal FHR pattern has also been reported in association with abruptio placentae and maternal administration of alphaprodine. When persistent, a true sinusoidal pattern may signify significant fetal compromise, and appropriate diagnostic or therapeutic maneuvers should be initiated. More commonly, however, a sinusoidal-like pattern is not of clinical significance.
Fetal Heart Rate Accelerations: Spontaneous and Evoked
Periodic intrapartum FHR accelerations (more than 14 bpm above baseline lasting more than 14 seconds and less than 2 minutes)14 may be spontaneous, associated with uterine contractions, or related to fetal movements. As in the setting of antenatal surveillance (e.g., nonstress test), intrapartum FHR accelerations provide evidence of intact fetal central nervous system function and, thus, fetal well-being. The presence of spontaneous FHR accelerations clearly is a reassuring sign. The converse, however, is not true; the absence of such accelerations is not necessarily worrisome, and other FHR tracing characteristics should be used to evaluate fetal status.
Intrapartum FHR accelerations also can be evoked with either scalp stimulation of vibroacoustic stimulation. Clark and associates20 reported that the presence of FHR accelerations (15 bpm for at least 15 seconds) after digital or instrumental fetal scalp stimulation uniformly was associated with a fetal scalp pH greater than 7.19. On a practical basis, the fetal scalp stimulation test is useful for evaluation of the fetus showing a nonreassuring FHR pattern, particularly when the cervix is not sufficiently dilated to permit fetal scalp sampling for pH determination. Smith and associates21 observed that similar FHR accelerations evoked by transabdominal vibroacoustic stimulation in the setting of nonreassuring FHR data were associated with a fetal scalp pH of greater than 7.25. The presence of evoked FHR accelerations is an excellent predictor of fetal well-being, although scattered reports have described isolated instances of fetal acidemia in fetuses with a reactive response to intrapartum vibroacoustic stimulation.22 It merits repeating that when confronted with nonreassuring FHR data, the entire intrapartum clinical setting needs to be assessed.
Fetal Heart Rate Decelerations
The evaluation of FHR decelerations arguably represents the most challenging aspect of FHR data analysis. Despite the myriad of classification systems proposed, we prefer to categorize these decelerations (typically, but not always, decreases of at least 10–15 bpm from the baseline value) simply as early, variable, or late in character. Most authentic disagreements about the analysis of FHR decelerations stem from differing assessments of the FHR baseline value. One unifying generalization typifies the discussion: The deeper the deceleration, the longer it lasts, the less variability present, and the greater the late component, the greater the chance that fetal compromise is imminent or already present. The types of FHR decelerations are described in what most authorities agree is the order of increasing potential concern.
Early decelerations display an onset, nadir, and recovery that is synchronous with the onset, peak, and end of the uterine contraction. In short, early decelerations mirror the uterine contraction pattern. Generally ascribed to fetal head compression (with resultant vagal stimulation), early FHR decelerations are the least common variety of intrapartum FHR deceleration observed and usually are of no clinical significance.
Early decelerations display an onset, nadir, and recovery that is synchronous with the onset, peak, and end of the uterine contraction. In short, early decelerations mirror the uterine contraction pattern. Generally ascribed to fetal head compression (with resultant vagal stimulation), early FHR decelerations are the least common variety of intrapartum FHR deceleration observed and usually are of no clinical significance.
Variable FHR decelerations are variable in shape as well as in timing with respect to the uterine contraction pattern. Characterized by an abrupt decline (defined as onset of deceleration to beginning of nadir less than 30 seconds)14 from the FHR baseline value and an equally abrupt recovery, variable decelerations display a variety of waveforms, such as V, U, W, or combinations (Fig. 2). The decline in FHR below baseline is more than 14 bpm, lasting more than 14 seconds and less than 2 minutes from onset to return to baseline.14 Although they may occur throughout the contraction cycle, this variety of deceleration occurs most frequently in the vicinity of the peak of a given contraction. A severe variant of the variable deceleration is defined by a decline of the FHR to less than 60 bpm (or a decrease of the FHR by at least 60 bpm from the baseline value) lasting 60 seconds or longer.
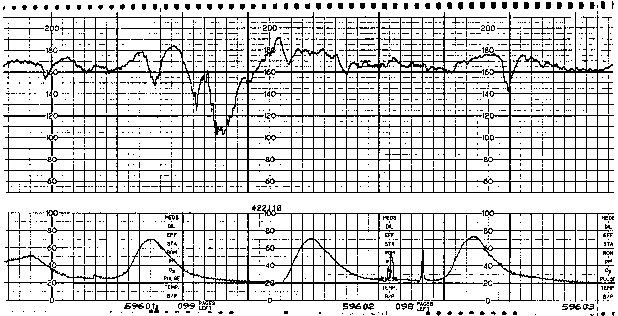 Fig. 2. Fetal heart rate pattern with variable heart rate decelerations and reassuring variability.
Fig. 2. Fetal heart rate pattern with variable heart rate decelerations and reassuring variability.
The etiology of variable decelerations is likely related to umbilical venous and arterial occlusion. Initially, with occlusion of the thin-walled umbilical vein, venous return to the fetal right atrium is reduced, producing a reflex tachycardia. This pattern often is observed as a shoulder on the FHR monitor strip immediately before the abrupt variable FHR deceleration. Shoulders that precede and follow variable decelerations are indicative of intact fetal central nervous system function and, thus, relative fetal well-being (Fig. 3). When cord occlusion becomes complete (including the umbilical artery), the low-resistance placental circulation is no longer in series. Instead, the elevated peripheral resistance that is caused by umbilical artery occlusion predictably leads to fetal hypertension, with subsequent baroreceptor stimulation. The baroreceptor and resultant vagal responses ultimately produce the parasympathetically mediated FHR deceleration. With relief of the umbilical cord occlusion, the sequence is reversed.
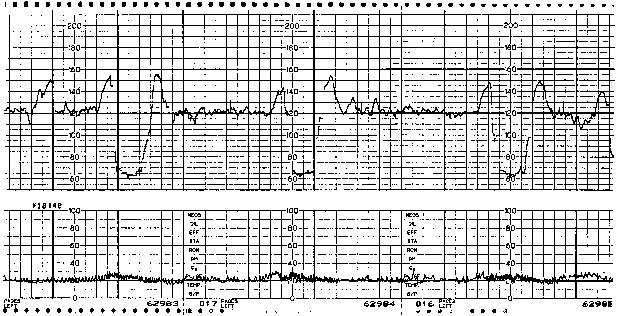 Fig. 3. Fetal heart rate pattern with repetitive variable fetal heart rate decelerations. Reassuring shoulders (accelerations) are obvious before and after each deceleration.
Fig. 3. Fetal heart rate pattern with repetitive variable fetal heart rate decelerations. Reassuring shoulders (accelerations) are obvious before and after each deceleration.
In isolation, variable FHR decelerations are not indicative of fetal compromise. If unremitting umbilical cord occlusion results in persistent, deep, variable FHR decelerations, however, the potential exists for the development of a fetal metabolic acidemia that cannot be managed satisfactorily by the fetal buffer system. Evidence of ensuing acidemia includes late recovery from the deceleration and decreased or absent interdeceleration variability as well as an ensuing bradycardia.23 Appropriate therapeutic interventions, including change of maternal position or amnioinfusion,24 may resolve the variable FHR deceleration pattern. The practice of amnioinfusion for the prevention or relief of umbilical cord compression, with resultant variable decelerations, is an accepted method of intrapartum management.24 Amnioinfusion has been reported to reduce the incidence of neonatal acidemia, to reduce the thickness of meconium, and to reduce the rate of operative intervention for nonreassuring FHR strips.25 Contraindications include persistent late FHR decelerations, fetal scalp pH less than 7.20, and significant vaginal bleeding; relative contraindications may include multiple gestation and malpresentation.
Amnioinfusion with normal saline (room temperature) can be accomplished via either bolus or continuous infusion using an infusion pump or gravity (intravenous bag 3–4 feet above the catheter). A bolus infusion of up to 800 mL may be administered, initially at a rate of 10–20 mL per minute until FHR decelerations resolve, and then up to an additional 250 mL to reach a maximum of 800 mL total. For a continuous infusion, a rate of 10 mL per minute is administered by infusion pump for 60 minutes, followed by an infusion at 3 mL per minute. Indications for discontinuation of the amnioinfusion include observation of a significant increase in the baseline intrauterine pressure, uterine hyperstimulation, or FHR bradycardia. Late FHR decelerations are characterized by a decline after the onset of the uterine contraction, a nadir after the peak of the contraction, and a slow recovery to the FHR baseline value after the end of the uterine contraction (Fig. 4). Late declarations have been described as being of two distinct etiologies. The first, caused by reflex, is mediated by the fetal central nervous system. The second, direct myocardial depression, is seen more often in a setting of metabolic acidemia. Classically ascribed to relative uteroplacental insufficiency, occasional or intermittent late decelerations are not at all uncommon during most labors. The presence of persistent late decelerations, however, must be carefully evaluated because they may be secondary to transient fetal hypoxemia in response to decreased placental perfusion associated with uterine contractions.
Late FHR decelerations are characterized by a decline after the onset of the uterine contraction, a nadir after the peak of the contraction, and a slow recovery to the FHR baseline value after the end of the uterine contraction (Fig. 4). Late decelerations have been described as being of two distinct etiologies.26 The first, caused by reflex, is mediated by the fetal central nervous system. The second, direct myocardial depression, is seen more often in a setting of metabolic acidemia. Classically ascribed to relative uteroplacental insufficiency, occasional or intermittent late decelerations are not at all uncommon during most labors. The presence of persistent late decelerations, however, must be carefully evaluated because they may be secondary to transient fetal hypoxemia in response to decreased placental perfusion associated with uterine contractions.
 Fig. 4. Fetal heart rate pattern with baseline value of approximately 140 bpm and repetitive late decelerations. These decelerations reach a nadir after the peak of the uterine contraction and then show a slow return to the fetal heart rate baseline value.
Fig. 4. Fetal heart rate pattern with baseline value of approximately 140 bpm and repetitive late decelerations. These decelerations reach a nadir after the peak of the uterine contraction and then show a slow return to the fetal heart rate baseline value.
The potential for fetal compromise in the presence of persistent late FHR decelerations mandates appropriate diagnostic and therapeutic interventions. Therapeutic maneuvers include maternal repositioning in the lateral recumbent position, satisfactory intravenous hydration, maternal oxygen therapy, and relief of uterine hyperstimulation, if present. The clinical index of suspicion for potential fetal compromise should be heightened if late FHR decelerations are accompanied by diminished variability or baseline fetal tachycardia. If palliative measures are unsuccessful and late FHR decelerations persist, an attempt to evoke a FHR acceleration via either digital examination or vibroacoustic stimulation, or a fetal pH determination via scalp sampling may be considered. Although isolated late FHR decelerations are not an indication for immediate cesarean section, persistent late FHR decelerations remote from the time of anticipated vaginal delivery often ultimately prompt operative abdominal delivery.
Finally, a prolonged deceleration is a decrease in the FHR from baseline of more than 14 bpm lasting more than 2 minutes and less than 10 minutes from onset to return to original baseline.14 A prolonged FHR deceleration lasting more than 10 minutes is defined as a baseline FHR change.
A combination of various types of FHR decelerations may occur. For instance, FHR decelerations may show an abrupt decline from the baseline value, with a nadir after the peak of the contraction followed by a slow recovery to the baseline level. These variable decelerations with a late component may appear in labors that are complicated by intermittent umbilical cord occlusion, fetal malposition, or cephalopelvic disproportion. Persistence of this pattern of FHR deceleration, with progressively longer recovery times to the baseline level, may be associated with an increased potential for fetal compromise. Clearly, it would be an error to consider only shallow, smooth decelerations with a slow return to the baseline value as late. An important principle of accurate fetal assessment is an appreciation of the timing and depth of any FHR deceleration, regardless of the type of FHR pattern that may have preceded it. The evaluation and treatment of variable FHR decelerations with a late component logically proceed as described above for classic late FHR decelerations.
The FHR decelerations observed with maternal pushing during the second stage of labor are not typically indicative of potential fetal compromise and usually are not an indication for immediate operative delivery. Despite their depth, which can be significant, these decelerations tend not to persist. Unless complicated by a protracted second stage of labor, development of a late component, loss of previously normal variability, or a prolonged deceleration, these FHR decelerations likely reflect fetal head compression, with resultant vagal stimulation, rather than evidence of uteroplacental dysfunction. Under ambiguous clinical circumstances, fetal scalp sampling for pH determination may be helpful to assess fetal status more accurately.
Although the NICHD guidelines do not recognize various "subtypes" or combinations of decelerations that have been historically referred to (such as "with a late component"), certainly very long decelerations, decelerations that are associated with a prolonged time to return to baseline, a rising baseline or tachycardia, and decelerations that are associated with a loss of variability between decelerations are concerning. There is evidence that these types of decelerations are associated with evolving hypoxia, and prompt delivery is indicated if intrauterine resuscitative methods fail to improve the FHR tracing.23 We agree with this approach. J. Parer et al. have suggested a 5-level color coded scale based on judging the risk of acidemia, which is another physiologic approach to FHT interpretation.24
Fetal Scalp Sampling
The potential applications of intrapartum fetal scalp sampling for pH determination have been discussed throughout this chapter. It is our impression that since the publication of previous editions of this chapter, the practice of scalp pH determination has become much less common on many labor and delivery units. In other centers, however, scalp pH determination remains a frequently used diagnostic tool. Regardless, scalp blood pH determination may, at times, be extremely helpful in the clinical decision-making process. This fetal assessment tool, however, is undeniably invasive and at least moderately uncomfortable for the patient. Therefore, perhaps even more than with most interventions, the clinician must provide the patient and her family with a thorough discussion of the rationale for its use and its potential risks and benefits.
Originally introduced by Saling and Schneider in 1962,27 fetal scalp sampling for pH determination preceded the development of continuous EFM for intrapartum fetal assessment. In current practice, fetal scalp sampling for pH survey typically is reserved for the determination of fetal status when FHR data are ambiguous or nonreassuring. The technique of scalp sampling requires a cooperative patient in either the lateral decubitus or lithotomy position with uterine displacement. The amniotic membranes must be ruptured and the cervix dilated at least 2–3 cm to allow the vaginoscopic cone to be placed firmly against the fetal scalp. The area to be sampled is swabbed clean, and silicone gel may be placed at the planned puncture site to facilitate beading of the blood. A 2-mm blade on a long handle is used to make a sharp stab incision. The free-flowing blood is collected in preheparinized capillary tubes. After collection of the blood, one end of the tube is plugged, and pressure is maintained with a swab on the incision site to facilitate hemostasis. Contraindications to fetal scalp sampling include maternal infectious processes (e.g., positive HIV status, hepatitis, or active genital herpes simplex virus) and maternal coagulation disorders (e.g., hemophilia, possibly idiopathic thrombocytopenic purpura).
Most authorities interpret a scalp pH of greater than 7.25 as reassuring. Values between 7.20 and 7.25 are considered borderline, and the test may be repeated in approximately 15 minutes, after a general re-evaluation of the entire FHR pattern and labor progress. Fetal scalp pH values below 7.20 are nonreassuring and are an indication to proceed with delivery by the most expeditious route. If significant doubt exists about the integrity of the specimen, or if the FHR pattern is patently inconsistent with an abnormal scalp pH value, an immediate repeat determination may be performed as preparations are being made to effect delivery. Additionally, an emerging technology, computerized cardiotogography with ST analysis appears to provide information equivalent to fetal scalp pH testing.28 Fetal scalp blood sampling appears to be declining in use, and currently many centers have stopped using this technique.
Continuous fetal pulse oximetry is an emerging technology for intrapartum fetal assessment. With transcervical placement of an infrared sensor against the fetal cheek, it is possible to monitor fetal oxygen saturation continuously and thus, potentially, identify the truly compromised fetus more accurately. A recent prospective, randomized, multicenter controlled trial of continuous intrapartum fetal pulse oximetry29 showed a greater than 50% reduction in the number of cesarean sections performed for nonreassuring fetal status in the study group. Interestingly, the overall cesarean birth rate was unchanged because of an increase in cesarean birth for dystocia in the study group. Although this promising assessment tool may improve clinicians' ability to identify acidemic and depressed fetuses, further study is needed before there is widespread application of this technology.
Fetal Pulse Oximetry
Continuous fetal pulse oximetry is an emerging technology for intrapartum fetal assessment. With transcervical placement of an infrared sensor against the fetal cheek, it is possible to monitor fetal oxygen saturation continuously and thus, potentially, identify the truly compromised fetus more accurately. A recent prospective, randomized, multicenter controlled trial of continuous intrapartum fetal pulse oximetry29 showed a greater than 50% reduction in the number of cesarean sections performed for nonreassuring fetal status in the study group. Interestingly, the overall cesarean birth rate was unchanged because of an increase in cesarean birth for dystocia in the study group. An interesting result of the studies on fetal pulse oximetry comes out of the FOREMOST trial group. This study revealed that fetal pulse oximetry would result in a cost savings of A$813 (approximately US$560) for each cesarean delivery averted.30 Unforunately, a recent meta-analysis confirmed that the addition of fetal pulse oximetry does not reduce cesarean section rates.31 Although this was once a promising assessment tool, a better method of monitoring fetuses in labor is still needed.
Computerized Cardiotogography
Recently, because of concerns about the poor positive predictive value, intra-and inter-observer reliability of fetal heart rate monitoring, computerized cardiotocography has started to come into the obstetricians' reperetoire. Initially, attempts were made to reduce inter- and intra-observer reliability by introducing computer analysis. Initial reports found that computerized analysis of the traditional FHR were not superior to those by physicians and midwives.16 The most recent advance in computerized fetal monitoring is STAN® (Noventa Medical). The STAN methodology combines conventional FHR analysis with analysis of ST changes thought to predict fetal hypoxia and acidosis.32
Computerized cardiotocography has been shown to have a NPV for a cord pH <7.10 of 97.9%.33 A recent Cochrane meta-analysis has shown that ST segment analysis was associated with fewer infants born with severe metabolic acidosis (RR 0.64, 95% CI 0.41–1.00), fewer fetal scalp blood samples in labor, fewer babies with neonatal encephalopathy (RR 0.33; 95% CI 0.11–0.95), fewer operative vaginal deliveries (RR 0.87; 95% CI: 0.78–0.96); but no reduction in cesarean section rates (RR 0.97; 95% CI: 0.76–1.01).34
Despite the initial promise of a reduction in unnecessary intervention from ST segment analysis, there have been some less positive reports. In one study, ST segment analysis was shown to have a sensitivity of <50% for metabolic acidosis at birth (although this study also confirmed the high negative predictive value of 96%). This study, however evaluated ST segment analysis as used apart from traditional fetal heart rate monitoring.35 There have been some isolated reports of newborns with severe metabolic acidosis at birth without significant ST changes, at least two of these newborns had "preterminal" fetal heart rate monitoring which necessitated intervention.36 Fetal scalp sampling for pH determination, fetal scalp stimulation, and vibroacoustic stimulation should not be considered in a vacuum. These data provide valuable clinical markers of fetal status, but comprehensive intrapartum fetal assessment requires an appraisal of the entire maternal-fetal clinical circumstance, including the stage of labor, the presence or absence of meconium-stained fluid, and the trends evident in the FHR data. Individual patient care is optimized when management decisions reflect the fact that no single parameter (clinical, electronic, or biochemical) is an infallible guide. The accomplished clinician will gather, analyze, and synthesize data that affect all of the pertinent intrapartum forces to maximize the efficiency and outcome of clinical decision-making.
Intrapartum EFM is not without risk. Serious complications are uncommon, but they can occur in both the mother and the fetus. The single greatest risk of continuous EFM is likely the increased rates of cesarean delivery and operative vaginal delivery associated with its use.7 The relationship between the use of continuous intrapartum EFM and the increased frequency with which nonreassuring fetal status is diagnosed is well established. Similarly, most studies have noted increased rates of cesarean delivery or operative vaginal delivery when continuous EFM was used, precisely because nonreassuring fetal status was diagnosed with greater frequency. These findings are not surprising, because the goal of intrapartum FHR monitoring is the detection of signs that may predict adverse events to facilitate intervention, and thus clinicians have shown an increasing willingness to intervene via cesarean section in an attempt to reduce the potential for fetal hypoxemic morbidity and mortality. It is difficult to determine accurately the extent to which medicolegal considerations contribute to the increased diagnosis of potential fetal compromise and the resultant increased rates of cesarean delivery.37 , 38 Experienced clinicians admit, however, that it is far more common to face litigation for failure to perform a cesarean delivery in a timely fashion than to face litigation for performance of an unnecessary cesarean delivery.
Uterine perforation can occur with the placement of the intrauterine pressure catheter. This risk can be minimized by the use of soft catheters, by avoiding the use of excessive force during placement, and by the use of an insertion technique that limits the possibility that the catheter guide will extend beyond the clinician's extended fingers. If perforation is suspected because of the absence of pressure changes with palpable uterine contractions or a positive change with a maternal Valsalva maneuver, the catheter should be removed immediately. After removal of the catheter, close maternal–fetal surveillance is indicated, but the necessity for surgical exploration (e.g., due to acute intra-abdominal hemorrhage) is rare.
Significant fetal hemorrhage after internal scalp electrode application or fetal scalp pH determination is uncommon. When hemorrhage follows scalp electrode placement, placental perforation or abruptio placentae should be suspected. Determination of the origin of such bleeding may be indicated by either Apt or Kleihauer–Betke testing. If bleeding persists after scalp puncture for pH determination after three contractions despite the application of pressure with long, cotton-tipped swabs, some authors advocate placing a small clip across the puncture site to achieve hemostasis.39 Persistent bleeding that occurs after any monitoring manipulation and is accompanied by nonreassuring FHR changes may be an indication for abdominal delivery if vaginal delivery is not imminent.
The invasive nature of intrapartum monitoring with an intrauterine pressure catheter, a scalp electrode, or scalp pH determination raises the possibility of maternal or fetal infectious complications. Neither maternal postpartum endometritis nor neonatal scalp infection is a common sequela.40 , 41 Neonatal scalp abscesses often are sterile, and they respond rapidly to conservative surgical drainage and local therapy. However, more serious scalp infections from FSE placement sites have been reported.42 There is little evidence that the rate of postpartum endometritis is elevated substantially in patients monitored invasively compared with patients monitored with external techniques undergoing vaginal delivery. Invasive fetal monitoring, however, should be avoided in patients with positive HIV status or hepatitis.
The use of intrapartum EFM may be met with resistance on the part of some patients and health-care providers. As reported by Jessica Mitford43 in a caustic best-seller condemning contemporary obstetric practices, "One of the most heartfelt objections (of mothers) to their hospital experience… was their coerced endurance of this tool of the obstetrician's trade." The anxiety and resistance related to intrapartum EFM may be attributed to a lack of, or incorrect, information. EFM is not incompatible with natural childbirth, and accurate information about the methods and rationale for fetal monitoring should be incorporated into prepared childbirth courses. Monitoring is a screening test that was designed to identify potential complications rather than a test to be used only when the fetus apparently is in trouble. Continuous EFM data, when properly interpreted and explained, can provide reassurance for the patient, her family, and the entire health-care team. Patient education that includes informational booklets and consultation with the nursing and physician staff will help answer many questions and decrease patient anxiety. Ideally, this educational process should begin early in the course of prenatal care.
It is important to remember that medications given intrapartum to the mother can also be transported across the placenta to the fetus, and may therefore have an effect on the intrapartum monitoring. Some commonly encountered examples are highlighted in the table below.
| Medication | Effect |
| Butorphanol (Stadol) | Transient sinusoidal pattern |
| Cocaine | No characteristic changes |
| Corticosteroids (such as betamethasone or dexamethasone) | Decrease in variability with betamethasone (not seen with dexamethasone) |
| Meperidine (Demerol) | No characteristic changes |
| Morphine | Decreased frequency of accelerations |
| Nalbuphane (Nubain) | Decreased frequency of accelerations, decreased variability |
| Terbutaline | Abolishment or decrease in frequency of late decelerations |
| Zidovudine (AZT) | No changes |
Adapted from ACOG Practice Bulletin Number 70: Intrapartum Fetal Heart Rate Monitoring44
Traditional neonatal assessment has used the Apgar score at 1 and 5 minutes as a means to identify infants who require further resuscitative efforts (1 minute) and to evaluate the results of those efforts (5 minutes).45 , 46 The components surveyed (each scored as 0, 1, or 2) to determine the Apgar score include heart rate, respiration, muscle tone, reflex irritability, and color. The Apgar score may be affected by a number of factors that are unrelated to intrapartum fetal hypoxemia or acidemia, including interobserver variability, prematurity, maternal sedation, neonatal cardiovascular compromise, structural anomalies of a major organ system, and central nervous system dysfunction related to an acute or chronic fetal injury that predated the labor process. The Apgar score is an excellent tool to assess neonatal status quickly, but it provides no insight into the potential etiology of any neonatal depression that may be evident.47 , 48 A large, population-based study of more than 1 million term babies did, however, conclude that a 5-minute Apgar score less than 7 was associated with an increased risk of neonatal morbidity, infant mortality, and neurologic dysfunction.49 Of particular concern was the finding that the OR for cerebral palsy was 31.4 (95% CI 27.3–36.1) in infants with 5-minute Apgar scores less than 7.
A more objective measure of the newborn's metabolic status is umbilical cord blood acid—base analysis. Most authorities agree that newborn assessment with the Apgar score is not interchangeable with newborn assessment with umbilical cord blood gas analysis.50 Fundamentally, the Apgar score addresses neonatal depression and, as noted above, can be affected by a number of factors that are unrelated to intrapartum fetal hypoxemia or acidemia. In contrast, umbilical cord blood gas analysis provides an objective evaluation of the newborn metabolic profile unrelated to the presence or absence of neonatal depression. Consequently, these measures assess separate, although related, components of newborn status. Not surprisingly, most authors have reported that only under extreme circumstances (e.g., Apgar score less than 4 at 10 minutes, umbilical cord arterial pH less than 7.0) does any significant correlation exist between Apgar scores and umbilical cord blood gas values.24 ACOG recommends that physicians obtain both venous and arterial cord blood in the following circumstances: cesarean delivery for nonreassuring fetal heart tracing, 5-minute APGAR score <7, severe growth restriction, abnormal fetal heart tracing, maternal thyroid disease, intrapartum fever and multifetal gestation.27 Because the documentation of fetal arterial and venous pH at birth, some clinicians obtain cord blood gasses at each delivery. Certainly in high risk situations, umbilical cord blood gasses can be helpful. Also, medicolegally the documentation of a normal pH at birth (with the high false-positive rate, most infants delivered for an abnormal FHT will have normal cord blood gasses) can protect a physician undergoing medicolegal proceedings. Also, the documentation of an abnormal umbilical cord blood gas, particularly if non-reassuring fetal status was the reason for delivery, can help to justify the decision to deliver the infant.
Some authorities advocate a policy of routinely obtaining umbilical cord blood gas analysis at the time of all deliveries. When performed on a routine basis, this practice may be an effective risk-management tool to document the absence of any significant metabolic acidemia in the immediate newborn period. Other units have adopted the policy of obtaining an umbilical cord blood gas analysis only when the intrapartum FHR tracing is worrisome, the infant is premature, or the condition of the neonate is depressed. The technique for performing umbilical cord blood gas analysis is straightforward and involves doubly clamping and dividing a 10- to 20-cm segment of umbilical cord immediately after delivery of the newborn. The doubly clamped umbilical cord may be left at room temperature for up to 60 minutes before sampling (while newborn assessment is conducted) without any significant change in pH values.50 An umbilical cord blood gas specimen that is drawn into a heparin-flushed syringe is stable for 30–60 minutes.
The definition of an abnormal umbilical artery pH value is problematic. The mean umbilical artery pH for term and premature infants is approximately 7.27–7.29, with a lower limit of normal (i.e., two standard deviations below the mean) of 7.13–7.15.24 More important, however, is the critical threshold for an abnormal umbilical artery pH. Currently, most authorities agree that clinically significant or pathologic acidemia is not present until umbilical artery pH values fall below 7.0 with a significant metabolic component (Table 1).24
Table 1. Classification of fetal or newborn acidemia
| Acidemia Type | Pco2(mmHg) | HCO3 -(mEq/L) |
| Respiratory | High | Normal |
| Metabolic | Normal | Low |
| Mixed | High | Low |
ACOG: Umbilical artery blood acid–base analysis: ACOG technical bulletin 216. Washington DC, ACOG, 1995
The ACOG issued a joint statement with the American Academy of Pediatrics outlining the stringent criteria that must be met before any causal relationship can be identified between intrapartum events and long-term neurologic dysfunction.47 , 48 , 51 Before any plausible causal link can be postulated, the following criteria must be present: profound umbilical artery metabolic or mixed acidemia (pH below 7.0); persistence of an Apgar score of less than 4 at 10 minutes; neonatal neurologic sequelae (e.g., seizures, coma, or hypotonia); and multisystem organ dysfunction (e.g., cardiovascular, gastrointestinal, hematologic, pulmonary, or renal system). Isolated pure intrapartum hypoxia accounts for only 4% of moderate to severe newborn encephalopathy (only a proportion of which will go on to develop cerebral palsy).37
A substantial body of data clearly shows that the contribution of intrapartum events to subsequent neurologic dysfunction generally is limited and certainly is difficult to determine with any certainty in all but a few specific cases.52 Nonetheless, the persistent misconception that intrapartum events are central to subsequent neurologic dysfunction is difficult to eradicate and should prompt the prudent clinician to obtain and document the appropriate objective information about newborn status as an integral component of a comprehensive risk-management program.
Source: https://www.glowm.com/section-view/heading/Intrapartum%20Fetal%20Monitoring/item/202
0 Response to "Continuous External Fetal Monitoring Involves the Use of a Spiral Electrode"
Post a Comment